Allogeneic chimeric antigen receptor (CAR) T cell therapies hold promise to improve CAR T access. However, limited expansion and persistence due to rejection in some recipients may impact efficacy. Elucidation of cellular mechanisms governing allogeneic CAR T expansion and persistence may inform patient management and technology development. We hypothesized that cell-extrinsic and intrinsic mechanisms differentially regulate rejection and expansion of allogeneic CAR T cells in patients with large B-cell lymphoma.
We identified 11 patients with relapsed/refractory large B-cell lymphoma treated on the ALPHA-2 phase 1 trial (NCT04416984) with ALLO-501A, a healthy donor-derived CD19 CAR T with T cell receptor (TCR) knockout. ALLO-501A uses Cellectis technologies. Despite treatment with a single ALLO-501A lot, we observed heterogeneity in CAR T expansion and clinical outcomes. Six patients had robust expansion (expanders), and five patients had relatively poor expansion (non-expanders) [median maximum CAR vector copy number 21,571 (range 10,296-104,784) versus 141 (range 55-502) copies/µg DNA, p < 0.01]. As with autologous therapies, expanders exhibited longer CAR T persistence and a higher frequency of clinical responses than non-expanders.
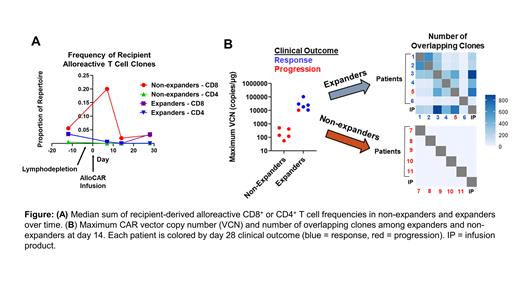
To elucidate cell-extrinsic mechanisms involved in allogeneic CAR T rejection, we hypothesized that recipient-derived alloreactive T cells limit expansion in non-expanders. We adapted a mixed lymphocyte reaction assay to identify alloreactive CD4 + and CD8 + T cell clones from pre-lymphodepletion PBMC samples. Briefly, labeled recipient PBMCs were incubated with irradiated ALLO-501A cells from the treatment lot. Proliferating recipient CD4 + and CD8 + T cells were isolated and underwent TCR sequencing. Alloreactive clones were defined as those enriched in this sample compared to an unstimulated sample from the same patient (p < 0.05 by Fisher's test) and were tracked post-treatment. Two expanders and two non-expanders were selected for analysis.
Non-expanders exhibited more robust in vitro T cell proliferation in response to ALLO-501A than expanders (85 vs. 40% proliferating; p = 0.03). Thus, more alloreactive clones were identified in non-expanders (median 762 CD4 + clones, 314 CD8 + clones) than expanders (median 313 CD4 + clones, 90 CD8 + clones). Non-expanders also had higher median alloreactive CD8 + T cell frequencies than expanders at days 7 and 14 following allogeneic CAR T infusion ( Figure A). A similar pattern was not observed for alloreactive CD4 + clones or non-alloreactive T cell clones, highlighting the potential role of recipient alloreactive CD8 + T cells in the rejection process and the need to control this process for the success of allogeneic CAR T.
Next, to define the role of cell-intrinsic mechanisms in allogeneic CAR T expansion, we tracked clonal evolution of the CAR T repertoire through TCR sequencing of the infusion product (IP) and post-treatment PBMC samples in all patients. At day 14 (peak allogeneic CAR T expansion), all expanders had numerous IP-derived clones and substantial clonotype overlap with each other. In contrast, no clonotype overlap was observed among non-expanders ( Figure B). Furthermore, we identified the same high-frequency IP-derived clones in multiple expanders and their relative abundance reflected the original clonal hierarchy of the IP. This pattern differs from autologous therapies, where clonal hierarchy is not maintained after infusion (Sheih, et al. Nat. Comm. 2020), and suggests that cell-intrinsic mechanisms may influence allogeneic CAR T expansion potential. Single-cell RNA and proteomic studies to characterize these expanding clones are ongoing.
In conclusion, we compared expansion kinetics across 11 allogeneic CAR T recipients to elucidate the cellular mechanisms governing allogeneic CAR T expansion and rejection. We revealed the impact of recipient alloreactive CD8 + T cells in allogeneic CAR T rejection and identified expansion of identical allogeneic CAR T clonotypes with retained clonal hierarchy in multiple patients. While patient factors such as tumor burden and target antigen expression likely play a role as well, our results suggest that strategies to eliminate alloreactive recipient cells and enhance allogeneic CAR T fitness through improved product design, manufacturing and lymphodepletion could improve allogeneic CAR T expansion, persistence and efficacy.
Disclosures
Jallouk:Kite, a Gilead Company: Consultancy. Kaimal:Allogene Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Furmanak:Allogene Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sommer:Allogene Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Lauron:Allogene Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sasu:Allogene Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Neelapu:Kite, A Gilead Company: Consultancy, Other: Advisory Board Member, Research Funding; Adicet Bio: Consultancy, Other: Advisory board member, Research Funding; Janssen: Consultancy, Other: Advisory board member; Morphosys: Consultancy, Other: Advisory board member; Incyte: Consultancy, Other: Advisory board member; Chimagen: Consultancy, Other: Advisory board member; Caribou: Consultancy, Other: Advisory board member; Astellas Pharma: Consultancy, Other: Advisory board member; Bristol Myers Squibb: Consultancy, Other: Advisory Board Member, Research Funding; Bluebird Bio: Consultancy, Other: Advisory board member; Sana Biotechnology: Consultancy, Other: Advisory board member, Research Funding; Fosun Kite: Consultancy, Other: Advisory board member; Allogene: Consultancy, Other: Advisory board member, Research Funding; Athenex: Consultancy, Other: Advisory board member; Sellas Life Sciences: Consultancy, Other: Advisory board member; Longbow Immunotherapy: Current holder of stock options in a privately-held company; N/A: Patents & Royalties: Related to cell therapy and the safety switch described (intellectual property); Precision Biosciences: Research Funding; Carsgen: Consultancy; Orna Therapeutics: Consultancy, Other: Advisory board member; Takeda: Consultancy, Other: Advisory board member; Synthekine: Consultancy, Other: Advisory board member; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; Merck: Consultancy, Other: Advisory Board Member. Bachireddy:BioNTech: Current equity holder in publicly-traded company; Exelixis: Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company; Breakbio Corp: Current equity holder in publicly-traded company; Allogene Therapeutics: Research Funding; Amgen: Current equity holder in publicly-traded company; Agenus: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal